The overall objective of Dr. Hirst’s research is directed at understanding the role of epigenetics in cancer and to investigate the therapeutic potential of interventions directed at epigenetic processes. He approaches this from an epigenomic perspective by combining innovative molecular biology and computational techniques with genome-wide detection platforms.
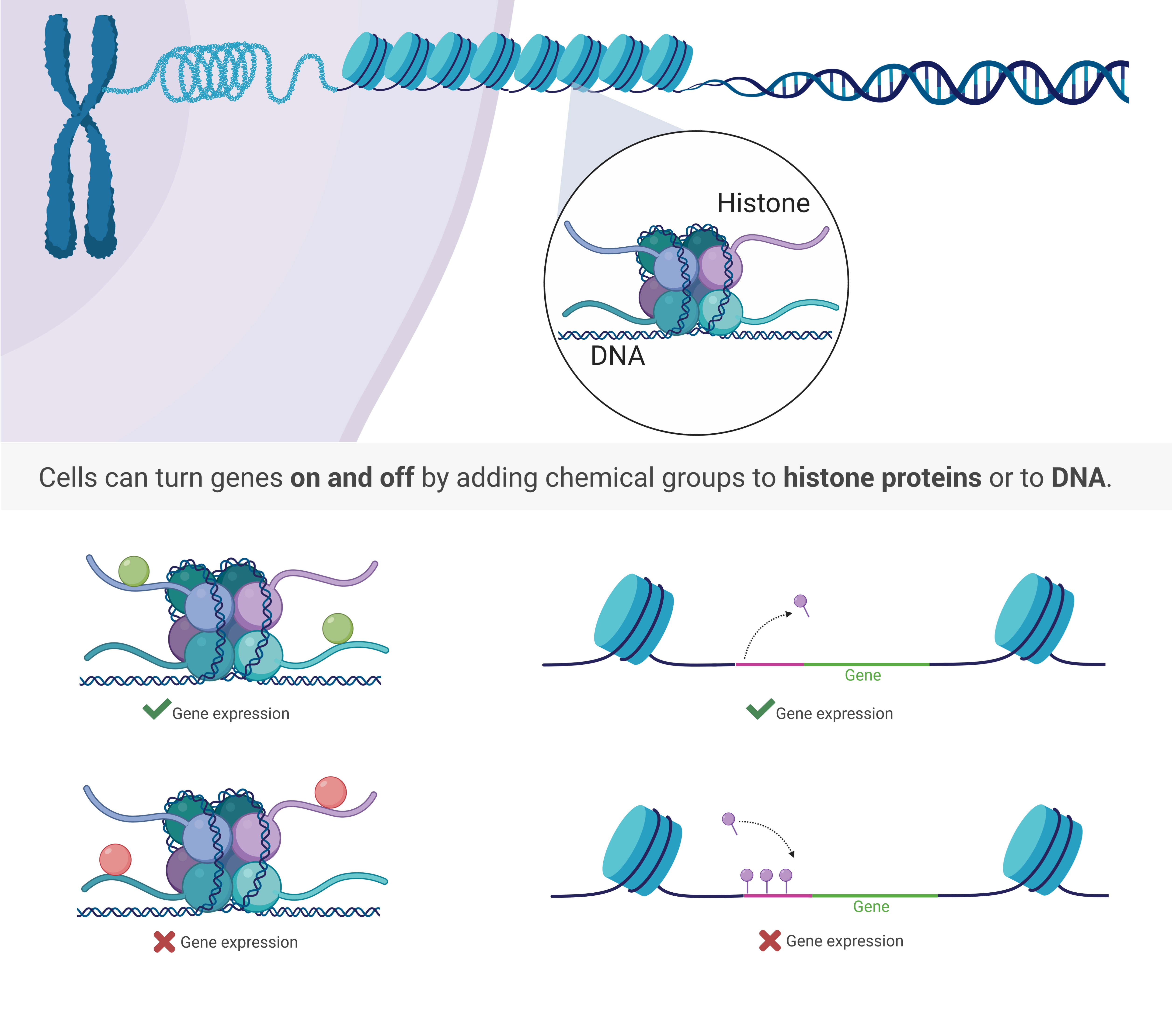
Epigenetics, the study of how covalent modifications to DNA and histones impact gene expression, is an emerging field with relevance to human disease. Normal cell development is accompanied by marked changes in the epigenome and specific epigenetic signatures have been identified in pluripotent, somatic and cancer cell types. Epidemiological and model organism studies have demonstrated that epigenetic modification can be induced via diverse environmental stimuli including stress, nutrient levels and toxin exposure. Epigenetic modification, which can be both transient and heritable in nature, thus provides a framework in which to investigate how environment and lifestyle choices impact disease susceptibility and progression. Furthermore, epigenetic modifications are central to chromatin dynamics and, as such, play key roles in many biological processes involving chromatin, such as DNA replication and repair, transcription and development. Our current understanding of the full repertoire of epigenetic modifications and the processes that they regulate is incomplete and we have only recently developed tools which allow for the study of normal human epigenomic polymorphism and the role that epigenetics plays in the initiation and progression of disease.