Genes Associated with Cell Sorting
tumours <- colnames(salmon$clean$counts)[grepl("^BL", colnames(salmon$clean$counts))]
salmon <- subset_salmon2(salmon, "tumours", "clean", genes = genes$no_mz,
patients = tumours)
colData(salmon$tumours$dds) <-
colData(salmon$tumours$dds) %>%
as.data.frame() %>%
mutate_if(is.factor, droplevels) %>%
as("DataFrame")
design(salmon$tumours$dds) <- formula(~ sex + SV1 + SV2 + SV3 + ebv_type +
clinical_variant + cell_sorting)
salmon$tumours$dds <- DESeq(salmon$tumours$dds, minReplicatesForReplace = 5)
salmon$tumours$de <- list()
salmon$tumours$de$cs <- list()
salmon$tumours$de$cs$lfc_0 <- results(
salmon$tumours$dds,
contrast = list(c("cell_sortingSorted"),
c("cell_sortingUnsorted")))
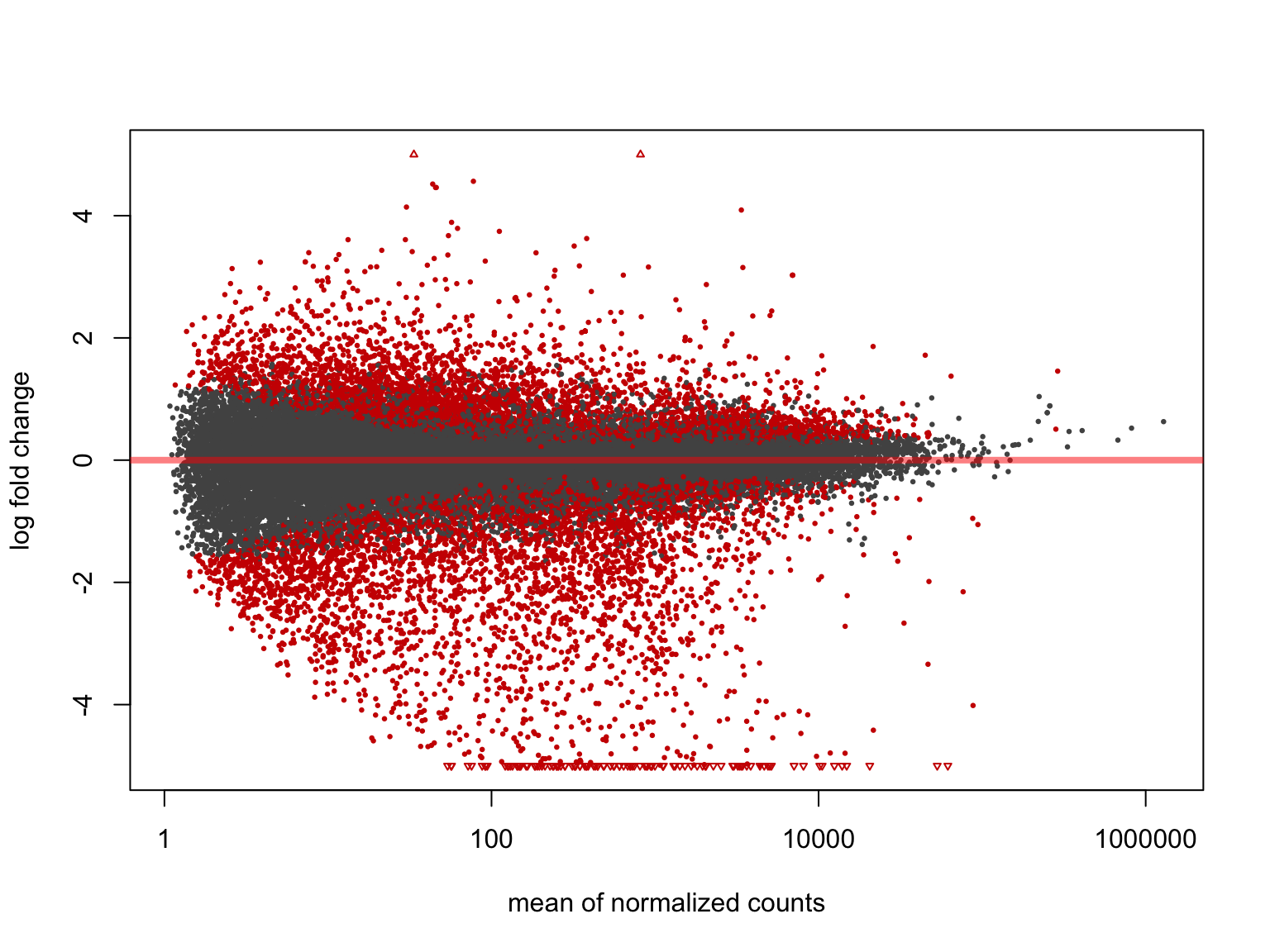
summary(salmon$tumours$de$cs$lfc_0)
out of 36687 with nonzero total read count
adjusted p-value < 0.1
LFC > 0 (up) : 4422, 12%
LFC < 0 (down) : 4342, 12%
outliers [1] : 0, 0%
low counts [2] : 0, 0%
(mean count < 1)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
plotMA(salmon$tumours$de$cs$lfc_0, ylim = c(-5, 5))
Model Fitting
clean_genes <- setdiff(genes$no_mz, get_sig_genes(salmon$tumours$de$cs$lfc_0))
design(salmon$clean$dds) <- deseq_design
salmon$clean$dds <- DESeq(salmon$clean$dds[clean_genes,],
minReplicatesForReplace = 5)
Tumour vs. Normal
salmon$clean$de <- list()
salmon$clean$de$tn <- list()
salmon$clean$de$tn$lfc_0 <- results(
salmon$clean$dds,
contrast = list(c("clinical_variantSporadic", "clinical_variantEndemic"),
c("clinical_variantCentroblasts", "clinical_variantCentrocytes")))
summary(salmon$clean$de$tn$lfc_0)
out of 27923 with nonzero total read count
adjusted p-value < 0.1
LFC > 0 (up) : 9956, 36%
LFC < 0 (down) : 8008, 29%
outliers [1] : 0, 0%
low counts [2] : 0, 0%
(mean count < 1)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
plotMA(salmon$clean$de$tn$lfc_0, ylim = c(-5, 5))

Endemic vs. Sporadic
salmon$clean$de$cv$lfc_0 <- results(
salmon$clean$dds,
contrast = list(c("clinical_variantEndemic"),
c("clinical_variantSporadic")))
summary(salmon$clean$de$cv$lfc_0)
out of 27923 with nonzero total read count
adjusted p-value < 0.1
LFC > 0 (up) : 2166, 7.8%
LFC < 0 (down) : 2690, 9.6%
outliers [1] : 0, 0%
low counts [2] : 0, 0%
(mean count < 1)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
plotMA(salmon$clean$de$cv$lfc_0, ylim = c(-5, 5))

EBV-positive vs. EBV-negative
salmon$clean$de$ebv$lfc_0 <- results(
salmon$clean$dds,
contrast = list(c("ebv_typeType.1", "ebv_typeType.2"),
c("ebv_typeNone")))
summary(salmon$clean$de$ebv$lfc_0)
out of 27923 with nonzero total read count
adjusted p-value < 0.1
LFC > 0 (up) : 2779, 10%
LFC < 0 (down) : 2133, 7.6%
outliers [1] : 0, 0%
low counts [2] : 542, 1.9%
(mean count < 2)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
plotMA(salmon$clean$de$ebv$lfc_0, ylim = c(-5, 5))

EBV Type 1 vs. EBV Type 2
salmon$clean$de$ebvt$lfc_0 <- results(
salmon$clean$dds,
contrast = list(c("ebv_typeType.1"),
c("ebv_typeType.2")))
summary(salmon$clean$de$ebvt$lfc_0)
out of 27923 with nonzero total read count
adjusted p-value < 0.1
LFC > 0 (up) : 17, 0.061%
LFC < 0 (down) : 21, 0.075%
outliers [1] : 0, 0%
low counts [2] : 542, 1.9%
(mean count < 2)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
plotMA(salmon$clean$de$ebvt$lfc_0, ylim = c(-5, 5))

Centroblasts vs. Centrocytes
salmon$clean$de$centro$lfc_0 <- results(
salmon$clean$dds,
contrast = list(c("clinical_variantCentroblasts"),
c("clinical_variantCentrocytes")))
summary(salmon$clean$de$centro$lfc_0)
out of 27923 with nonzero total read count
adjusted p-value < 0.1
LFC > 0 (up) : 986, 3.5%
LFC < 0 (down) : 1336, 4.8%
outliers [1] : 0, 0%
low counts [2] : 1083, 3.9%
(mean count < 2)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
plotMA(salmon$clean$de$centro$lfc_0, ylim = c(-5, 5))

Gene-based Differential Gene Expression
tfs <- c("TFAP4", "DDX3X", "ARID1A", "SMARCA4")
maf_patients <- unique(maf@data$patient)
salmon <- subset_salmon2(salmon, "muts", "clean", patients = maf_patients)
# Add mutation information to colData
colData(salmon$muts$dds) <-
maf@data %>%
filter(
is_nonsynonymous(Consequence),
patient %in% rownames(colData(salmon$muts$dds))) %>%
select(patient, Hugo_Symbol) %>%
distinct() %>%
mutate(status = "Mutated") %>%
spread(Hugo_Symbol, status, fill = "Unmutated") %>%
arrange(match(patient, rownames(colData(salmon$muts$dds)))) %>%
select(one_of(tfs)) %>%
mutate_all(as.factor) %>%
cbind(colData(salmon$muts$dds), .) %>%
as.list() %>%
map_if(is.factor, droplevels) %>%
DataFrame()
muts_design <- paste0("~ sex + SV1 + SV2 + SV3 + ebv_type + clinical_variant + ",
paste(tfs, collapse = " + "))
design(salmon$muts$dds) <- as.formula(muts_design)
salmon$muts$dds <- DESeq(salmon$muts$dds, minReplicatesForReplace = 5)
TFAP4
salmon$muts$de$tfap4$lfc_0 <- results(
salmon$muts$dds,
contrast = list(c("TFAP4Mutated"),
c("TFAP4Unmutated")))
summary(salmon$muts$de$tfap4$lfc_0)
plotMA(salmon$muts$de$tfap4$lfc_0, ylim = c(-5, 5))
DDX3X
salmon$muts$de$ddx3x$lfc_0 <- results(
salmon$muts$dds,
contrast = list(c("DDX3XMutated"),
c("DDX3XUnmutated")))
summary(salmon$muts$de$ddx3x$lfc_0)
plotMA(salmon$muts$de$ddx3x$lfc_0, ylim = c(-5, 5))
SWI/SNF Complex
salmon$muts$de$swisnf$lfc_0 <- results(
salmon$muts$dds,
contrast = list(c("ARID1AMutated", "SMARCA4Mutated"),
c("ARID1AUnmutated", "SMARCA4Unmutated")))
summary(salmon$muts$de$swisnf$lfc_0)
plotMA(salmon$muts$de$swisnf$lfc_0, ylim = c(-5, 5))





